Secure Online Forms to Run Clinical Trials
Advance healthcare with 123FormBuilder’s HIPAA-compliant forms—streamlining research, ensuring data security, and empowering collaboration for a healthier future.
Healthcare Service Providers That Count On 123FormBuilder




Run better-planned clinical trials
You’re collecting some type of data at every step of the clinical trial process. But the complex nature of clinical trials often makes it an inefficient, lengthy, and costly process. Using digital tools like 123FormBuilder is a powerful way to minimize some of these burdens by streamlining data collection. With our digital forms, you can recruit patients online, sign them up for your medical research, collect health data, or obtain electronic consent. By moving these critical operations online, you keep patients’ frequent visits to your facility to a minimum and, as a result, reduce the drop-out rate.
123FormBuilder lets you build both simple and highly complex studies in minutes with our intuitive drag & drop tool and then share the forms securely across teams or with your clinical trial participants. Running virtual clinical trials helps you expand the recruitment potential, collect more accurate data, better plan resources (like easily matching a patient with a facility), prevent delays, and improve the overall experience of the trial.

Secure your research with HIPAA compliance
By serving healthcare clients in the largest markets in the world, we know how to address the nuts and bolts of the security protocols specific to running clinical trials. 123FormBuilder ensures compliant data collection through our HIPAA-compliance that serves as a gatekeeper for all the PHI (Protected Health Information) you’re handling through our forms.
Rely on our advanced security measures that aim to mitigate security risks, build trust and protect the privacy of your patients and your research data at every step of the clinical trial. We count on AWS (Amazon Web Services) for data residency in the US and Europe, ISO 9001 & ISO 27001 Certifications, full GDPR compliance, and robust HIPAA security protocols to have you covered from every standpoint. Our ultimate goal is to create a safe space where you can focus, stress-free, on medical innovation.

Rely on experience and commitment
Each clinical trial has its particularities, which we understand and welcome. That’s why a Dedicated Account / Technical Manager will guide you through your work with 123FormBuilder and sit down with you in the search for solutions. We help you help the world, one form at a time.
To support you in increasing patient engagement while ensuring effective, accurate, and uninterrupted data collection, our form builder, integrates with 3rd party digital tools like Salesforce. You can easily map form fields to Salesforce objects and eliminate the manual work that slows things down and causes human error. Automate your clinical trial management and gain better visibility into your program.

Clinical Trials Workflows to Handle with HIPAA-compliant Forms

Automate operations
Whether it’s patient recruitment, collecting consent, or evaluating participant’s eligibility, use 123FormBuilder’s HIPAA compliant forms to automate key data collection workflows.
Ensure regulatory compliance
Stay GDPR and HIPAA compliant every step of the way by using online forms that adhere to advanced security protocols that protect patient and clinical research data.

Streamline patient onboarding
Build flexible patient scheduling, preauthorization, and preapproval processes. Schedule patient visits online, review their data and assign them to the closest medical facility.

Collect electronic consent
Obtain informed consent from your clinical trial participants by including a signature field that allows subjects to sign the document digitally.
Pass data between systems
Run a cost-efficient and timely clinical trial by connecting digital tools and pulling data from multiple external systems of record to provide a single view of patient information.

Track data & performance
Generate clinical report studies, evaluate patient data and program enrollment, and track documentation to overview the clinical trial’s performance thoroughly.
Secure Forms for Healthcare
We’re here to help you select the ideal plan for your business.
Just fill out the form, and we’ll:
Let’s connect and build the right solution together!
Pre-built Medical Form Templates You Can Use Right Away
An extensive gallery of ready-to-use online medical forms to help you get started. Choose yours.
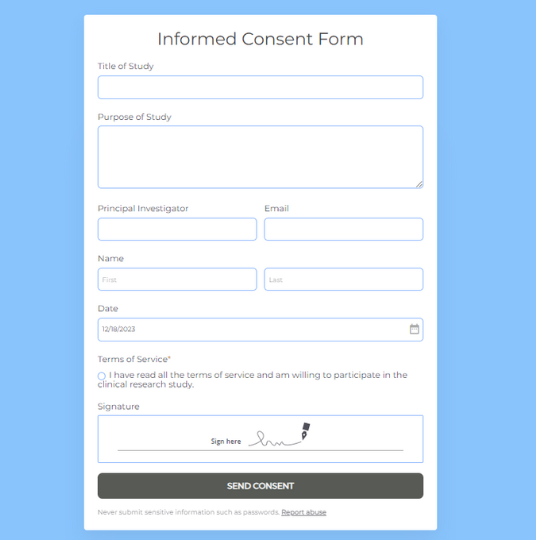
Informed Consent Form
Ensure ethical and transparent participation in your research or medical procedures with 123FormBuilder’s Informed Consent Form.
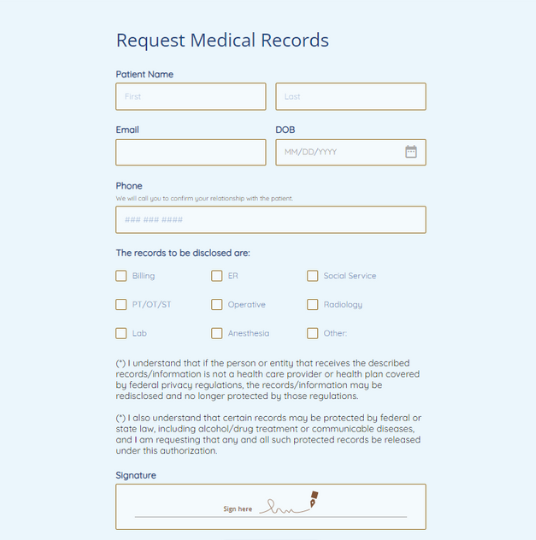
Medical Records Request Form
Requesting medical records is now simplified and secure, thanks to our intuitive Medical Records Request Form by 123FormBuilder.
Laboratory Test Request Form
Streamline your testing process with our Laboratory Test Request Form.
Medical History Questionnaire
Gather the medical history of your patients using this questionnaire template.
What It’s Like to Work with Us
My business provides health care services to clients in the home setting. We utilize 123FormBuilder for our employees to sign in from the client’s home and document the services they provide on their phones. I couldn’t be happier with their product.